- Product Details
Keywords
- high purity Tafluprost ethyl amide
- Tafluprost ethyl amide low price
- Tafluprost ethyl amide Manufacturer
Quick Details
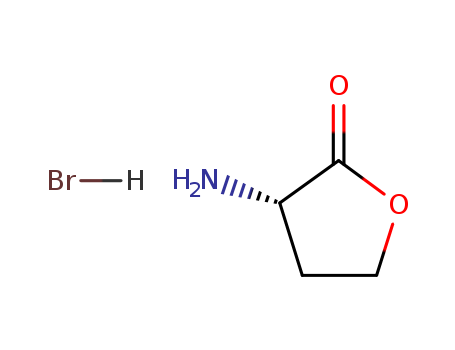
- ProName: CAS NO.1185851-52-8/Tafluprost ethyl a...
- CasNo: 1185851-52-8
- Molecular Formula: C24H33F2NO4
- Appearance: Colorless to pale yellow liquid
- Application: An important raw material and intermed...
- DeliveryTime: prompt
- PackAge: According to customer requirements
- Port: shanghai
- Purity: 98%Min
- Storage: Stored in the dry and and ventilated p...
- Transportation: By Sea,by Air,By courier like DHL or F...
- LimitNum: 0
- Moisture Content: N.M.T. 0.1%
- Impurity: N.M.T. 2%
Superiority
Our Quality system
We has a sound quality management system and documents, equipped with SHIMADZU HPLC, GC chromatography and other series of testing equipment, in order to meet the quality priority principle. Quality management department is independent of the production system, and execute with quality inspection and quality supervision, quality management functions.
The QA department is responsible for establishing and perfecting the quality supervision system, and set up the QA personnel from supplier evaluation of material, raw material procurement, warehousing acceptance, production, release, sales, customer feedback to monitor the whole process, further standardize and improve the quality management system; at the same time, we do on-site inspection and regular quality report and other means to manage the quality system, and regularly organize GMP training to update employee’s knowledge and improve the quality concept. We are strictly implementing GMP management, and are open to accept external customer audit and official inspections.
Our R & D
We use the advantage of combination of chemical synthesis and purification technology platform, to carry out research and development of various APIs and intermediates with high degree of difficulty, especially chiral drugs.
Our R & D team has more than 109 year-experience in R & D and scale production of prostaglandins, anti-cancer drugs and hypoglycemic drugs. All of these products have achieved commercial production, and occupied a leading position in the market competition.
Details
Shanghai Genriver Pharmaceutical Technology Co., Ltd. is a technology oriented company, see the quality as our life and dedicated to meet the customer’s requirement and provides a full range of services to customers as per GMP compliance, such as customized manufacturing, technology consultation, documentation services.
Based on the chemical synthesis and purification technology platform, we have a high level R&D standard laboratory for synthesis and also a multiple-function pilot production workshop and equipped with a full set of testing facilities.
We always adhere to the ""credibility first, quality first"" corporate creed, see the quality as life, and implementing GMP management, to provide customers with comprehensive services.
"





![Pyrazolo[1,5-a]pyrimidin-7(4H)-one, 3-(1-cyclohexen-1-yl)-6-(4-methoxyphenyl)-2-phenyl-5-(2-pyridinylamino)](http://file1.lookchem.com/cas/2023/08/24/2201056-66-6.jpg)
![Ethyl (S)-2-[3-[(2-Isopropyl-4-thiazolyl)methyl]-3-methylureido]-4-morpholinobutanoate Oxalate](http://file1.lookchem.com/300w/synthetic/2022-01-23-01/c42fc2d2-6547-46ef-be02-a470bae37a7e.png)